Medical & Pharmaceutical Automation Excellence
Motionwell delivers automation solutions for medical device and pharmaceutical manufacturers in Singapore and the region, with a focus on precision assembly, cleanroom-compatible equipment, and compliance-ready engineering. We design for stable production, validated documentation, and long-term maintainability on the factory floor.
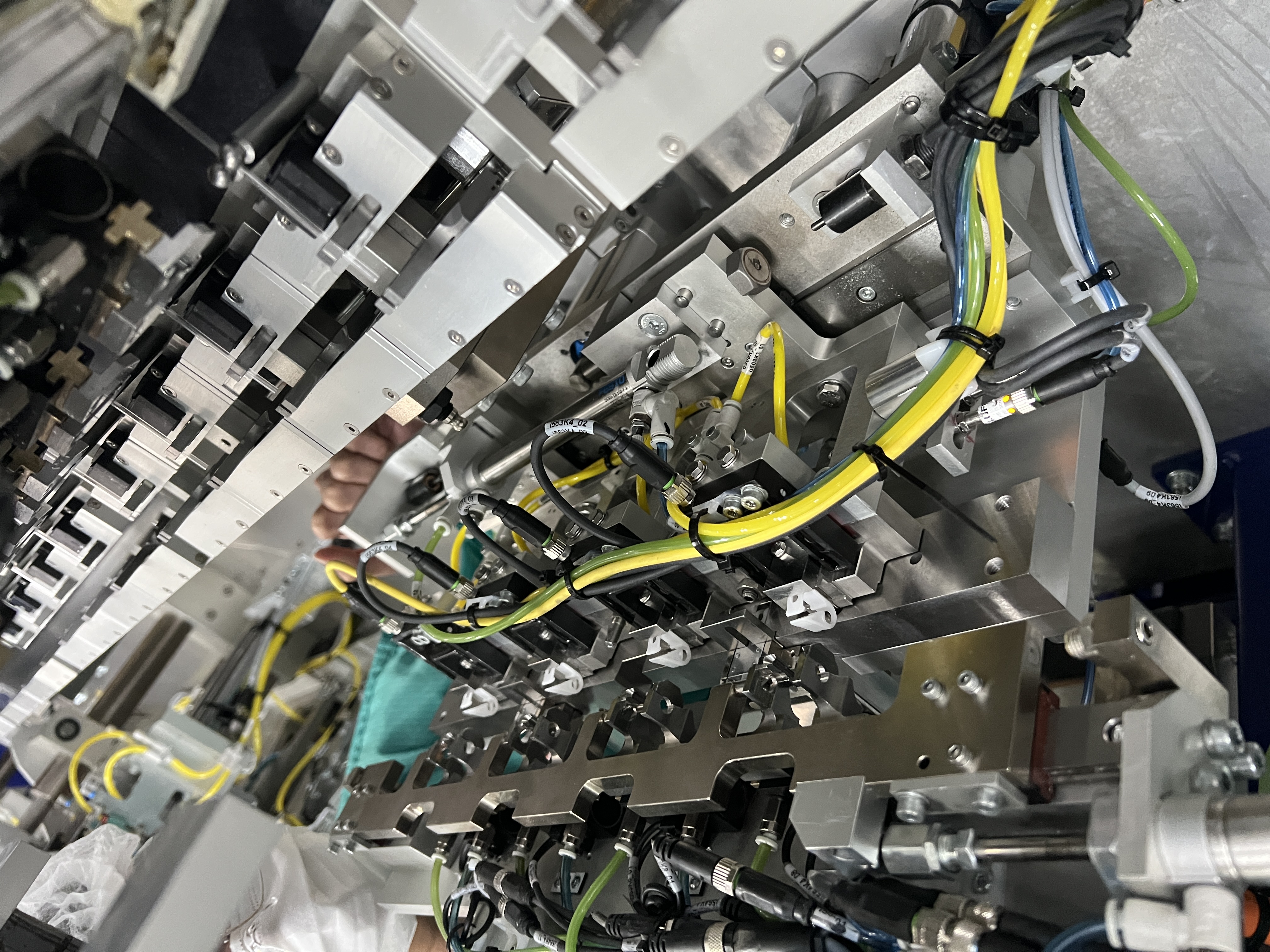
Automation Scope Across the Medical Value Chain
| Area | Typical scope | Common building blocks |
|---|---|---|
| Material handling | Feeding, singulation, tray loading, inter-station transport | Vibratory bowl feeders, linear tracks, vision-guided robotics, AMR/AGV logistics |
| Process and assembly | Press-fit, ultrasonic welding, torque fastening, dispensing | Servo transfers, pneumatic tooling, precision dispensing valves, recipe control |
| Inspection and testing | Cosmetic inspection, OCR/OCV, functional tests | Industrial cameras, strobe lighting, leak/pressure rigs, traceability logging |
| Serialization and coding | Batch/lot and track-and-trace workflows | TIJ/laser printing, vision verification, reject handling, HMI reporting |
| End-of-line | Primary/secondary packaging and warehouse handoff | Blister packaging, cartoning, case packing, palletizing, ASRS integration |
Project References From Motionwell Deliveries
Below are project references used as concrete examples for system scoping and design discussions.
| Project reference | Scope (high-level) | Key technologies | Why it matters |
|---|---|---|---|
| Project 0023 (Pharmacy Automation) | Serialization and vision inspection line with reject handling | Coding + OCR/OCV verification, HMI, pneumatic reject | Compliance-driven traceability and repeatable inspection |
| Project 0010 (Medical Pick & Place) | Automated pick-and-place and packaging transfer workflows | Starwheel indexing for vial handling, gang pick & place, vacuum EOAT, sensors | Gentle handling and stable indexing for high-volume medical parts |
| Rotary indexing assembly program | High-throughput assembly with integrated vision and sorting | 12-station rotary indexing, BD-standard vision checks, Epson SCARA sorting | Compact footprint, repeatable assembly, and fast station-to-station transfer |
Featured Build: Medical Rotary Assembly Machine
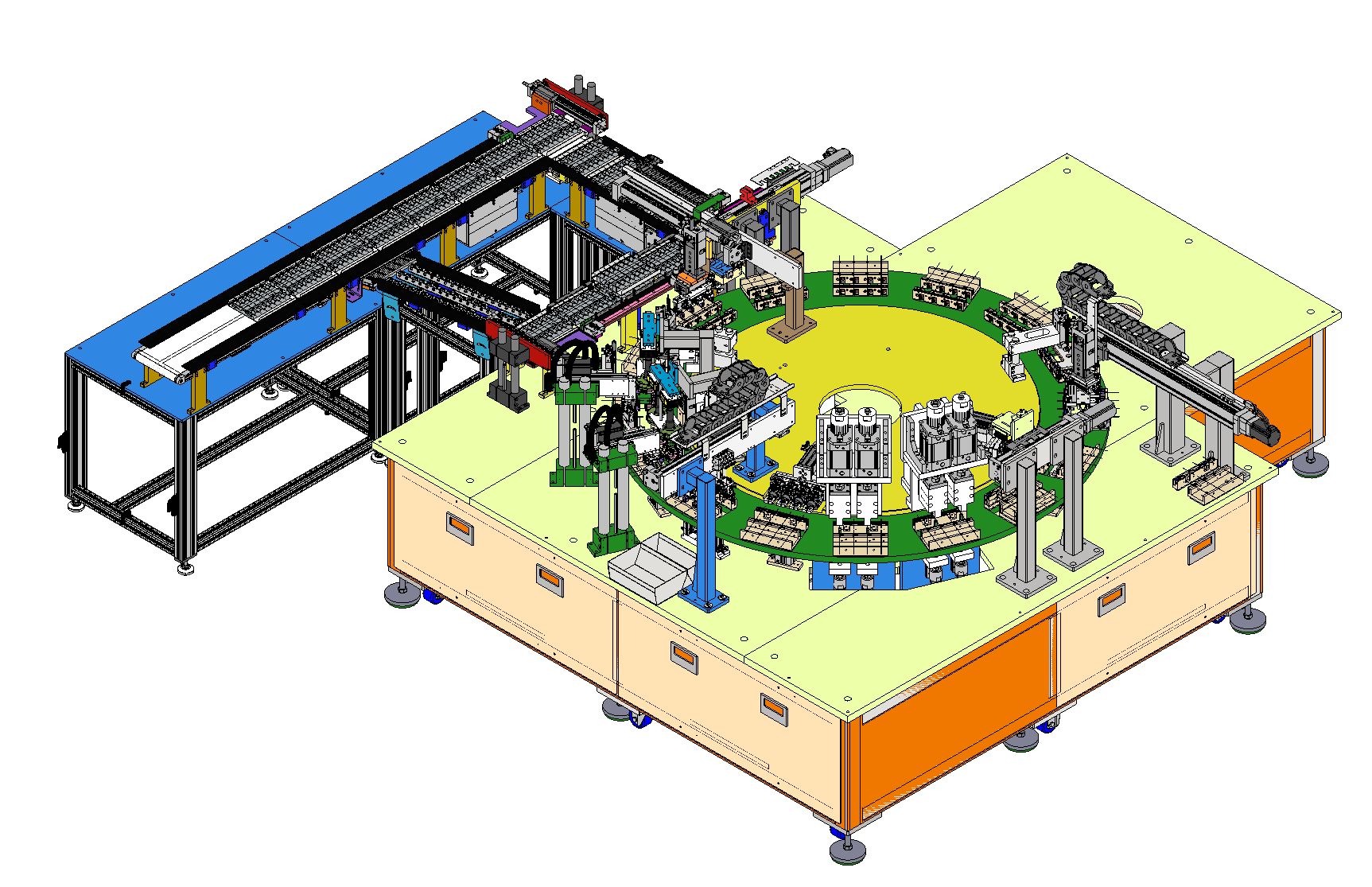
| Feature | Implementation detail | Outcome |
|---|---|---|
| High-throughput indexing | 12-station rotary indexing table | Stable cycle-to-cycle repeatability |
| Vision inspection standard | BD-standard vision inspection with OK/NG sorting logic | Consistent quality criteria and sorting control |
| Fast robot sorting | Epson SCARA robot for high-speed placement | Efficient downstream handling and separation |
| Cleanroom-ready design | Materials and design choices aligned to ISO Class 7/8 environments | Easier cleaning and controlled contamination risk |
Technical Specifications
| Parameter | Specification |
|---|---|
| Configuration | 12-station rotary indexing table |
| Vision system | BD-standard compliance, contour inspection |
| Robot | Epson SCARA for sorting and placement |
| Environment | ISO Class 7/8 cleanroom compatible |
| Integration | Vibratory feeding, servo transfer, pneumatic assembly |
Compliance, Validation, and Documentation
Medical and pharma projects succeed when engineering, documentation, and validation expectations are aligned early. We support compliance-driven delivery in a practical way.
| Requirement area | Typical scope | Typical deliverables |
|---|---|---|
| Quality systems | ISO-aligned build and service practices | Design records, change control, FAT/SAT evidence |
| Cleanroom compatibility | ISO Class 7/8 design constraints (project-dependent) | Material selection notes, cleaning instructions, layout considerations |
| Validation | IQ/OQ/PQ support (project-dependent) | URS/FS/DS, IQ/OQ protocols, execution records |
| Electronic records | 21 CFR Part 11 style traceability patterns (when required) | User roles, audit trail strategy, data retention approach |
| Functional safety | CE/UL/ISO 13849 approach (project-dependent) | Risk assessment, safety circuit design, safety test checklist |
Integration and Data Traceability
| Integration layer | Typical protocol options | Example usage |
|---|---|---|
| Machine control | PROFINET, Ethernet/IP | Station handshakes and recipe control |
| Data collection | OPC-UA, file export, API gateways | Production records, inspection images, batch reports |
| Enterprise systems | MES/ERP interfaces (project-specific) | Batch/lot reconciliation and production reporting |
Frequently Asked Questions
| Question | Answer |
|---|---|
| What cleanroom class can your equipment operate in? | Many medical programs target ISO Class 7/8 environments. The final design depends on your cleaning SOP, materials selection, and how utilities and airflow are managed around the equipment. |
| Do you provide validation documentation? | Yes. We can support FAT/SAT evidence and provide IQ/OQ deliverables when the validation scope is defined, including URS/FS/DS and protocol templates aligned to your quality system. |
| Can you integrate with existing MES/ERP systems? | Yes. We typically define the traceability model first (IDs, timestamps, state transitions), then implement a stable interface using OPC-UA, industrial Ethernet, or an agreed file/API exchange method. |
| What is your typical project timeline? | Medical automation timelines vary by validation depth and tooling complexity. A common range is 4–8 months from design kickoff through commissioning, with additional time for full validation where required. |
| Can you support UDI, coding, and serialization workflows? | Yes. We have project references that include coding, OCR/OCV verification, reject handling, and operator HMIs. The critical factor is defining your compliance rule set and data retention requirements up front. |
To discuss a medical or pharmaceutical automation project in Singapore, contact us at /contact/ with your product flow, target cleanroom class, and validation expectations.
Related capabilities: Material Handling | Process & Manufacturing | Inspection & Testing | Compliance & Standards
